Home Improvement
Everything you need to know about the sacrificial anodes and corrosion mitigation for civil structures
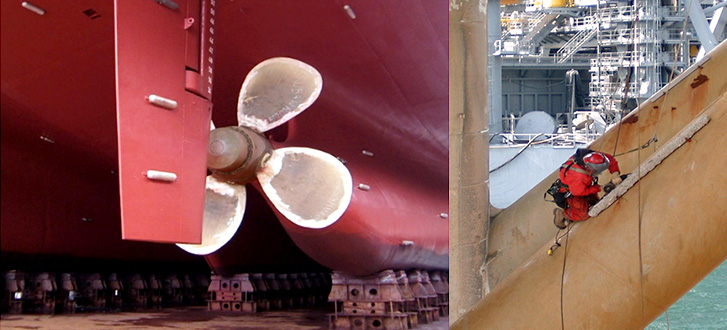
The sacrificial anode, also known as the galvanic anode, is the defence mechanism you need to prevent corrosion. Although it does not completely stop corrosion but they sacrificed themselves for it. As the name suggests, a sacrificial anode is a material that professionals install in pipes or tanks to sacrifice corrosion resistance.
How does a sacrificial anode work?
The way a sacrificial anode works is the same as how an electrochemical cell works. The Sacrificial Anodes india has a type of protective metal on the cathode side that is the negatively charged side of the device. The more reactive metal or alloy is on the anode or positive side. It is important to note that the metal or alloy on the anode side has a greater potential difference than the metal on the cathode side. When these two metals are in place, the reaction will occur naturally.
An oxidation reaction takes place at the anode. Oxidation means that the substance loses electrons. While this was happening, the reduction reaction took place on the cathode side this means that the substance will gain electrons. Producing both an oxidation reaction and a reduction reaction is called a redox reaction. Oxidation on the anode side ensures that the sacrificial metal will corrode. The reduction reaction at the cathode signal prevents the metal on that side from eroding.
A sacrificial anode can protect a large number of different metals from corrosion.
- Hulls in ship
- Distillery
- Pipeline
- Water heater
- Underground tank
- Distribution system
Corrosion Prevention: How to protect building structures from environmental damage?
The methods of the Corrosion mitigation for civil structures are given by,
Selections of materials also prevent corrosion in building structures:
Corrosion is caused by environmental factors such as humidity and air salinity. However, the use of certain materials can limit or completely negate the damage caused by these conditions.
Strategic uses of rustproof steel:
Iron and steel are prevalent in almost all construction applications. However, some alloy steels have limited corrosion resistance due to their high carbon content. Choosing a low carbon steel alloy, such as 300 series stainless steel, can reduce the potential for corrosion damage. However, the structural strength characteristics are reduced, and the price tends to rise. For this reason, construction projects with less anti-corrosion requirements tend to use the less expensive 400 series stainless steel.
Coated on top to prevent rust:
Many components can be identified with anticorrosive coatings. These come in several types with different properties. Epoxy coating provides good strength but is susceptible to colour fading. Alkyd coatings are similar in strength but are less susceptible to UV damage to the paint. Finally, polyurethane coatings tend to have the best strength and UV resistance characteristics.
Warranty inquiry:
Some component manufacturers state their parts warranties regarding corrosion resistance. However, it’s good to ask the supplier about any potential warranties. This is because it is a good indicator of both product performance and supplier confidence.
Using external protective equipment:
To reduce costs but improve rust protection in harsh environments, various coatings can be used with building components (typically steel) to prevent corrosion.
Home Improvement
Kyle Rittenhouse is acquitted of all charges in the trial over killing 2 in Kenosha

Kyle Rittenhouse, the 18-year-old who fatally shot two people during the unrest last year in Kenosha, Wis., has been acquitted of all charges in a criminal trial that divided the nation over questions about gun rights, violence at racial justice protests and vigilantism.
The verdict, delivered Friday, follows a highly watched trial in which prosecutors struggled to overcome Kyle Rittenhouse claim that he acted in self-defense on the night of the shootings.
“He has a huge sense of relief for what the jury did to him today. He wishes none of this would have ever happened, but as he said when he testified, he did not start this,” said Rittenhouse’s defense attorney Mark Richards, speaking to reporters outside the courthouse. “To say that we’re relieved would be a gross misunderstatement.”
In two weeks of testimony and evidence — led by a daylong turn on the stand by Kyle Rittenhouse himself — defense attorneys were able to convince the jury of 12 that the night of Aug. 25, 2020, was filled with deadly peril for the then-17-year-old.
Jurors deliberated for roughly 27 hours over the course of four days before pronouncing Rittenhouse not guilty on all five counts: first-degree intentional homicide, first-degree reckless homicide, first-degree attempted intentional homicide and two counts of first-degree reckless endangerment. The jury was also asked to consider lesser versions of several counts, but were not swayed.
“While we are disappointed with the verdict, it must be respected. We are grateful to the members of the jury for their diligent and thoughtful deliberations,” said the Kenosha County District Attorney’s Office in a statement. “We ask that members of our community continue to express their opinions and feelings about this verdict in a civil and peaceful manner.”

Prosecutors declined to give further comment.
A chaotic night of unrest in Kenosha turns deadly
Kyle Rittenhouse armed himself with an AR-15-style rifle on a night of unrest in Kenosha sparked by the police shooting of Jacob Blake, a 29-year-old Black man who was left paralyzed after an encounter with a white officer. Rittenhouse, who lived across the state line in Antioch, Ill., testified that he intended to act as a medic and help protect private property.
But the night spiraled out of control. In a series of chaotic encounters with protesters, documented thoroughly by photographs and videos, Kyle Rittenhouse shot and killed Joseph Rosenbaum, 36, and Anthony Huber, 26, and injured Gaige Grosskreutz, then 26.
During the trial, Kyle Rittenhouse said he feared for his life in all three cases. Rosenbaum, he said, had chased him and was grabbing for his rifle.
“Mr. Kyle Rittenhouse was chasing me. He said he was going to kill me if he got me alone. I was alone. I was running from him. I pointed it at him, and it didn’t stop him from continuing to chase me,” Rittenhouse testified.
Afterward, as he ran toward police, others, including Huber and Grosskreutz, began to chase him. Huber struck him with a skateboard, visual evidence confirms. Grosskreutz was holding a loaded Glock pistol, which, he admitted during cross-examination, was pointed at Rittenhouse, though he said that was unintentional.
The criminal trial ends with acquittal, but civil lawsuits could be coming
Prosecutors argued that Rittenhouse was responsible for creating those situations. He chose to bring a deadly rifle into a dangerous environment, they said, and chose to stay there even after being separated from a friend.
Rosenbaum was unarmed, they pointed out, yet Rittenhouse shot him four times, all as Rosenbaum was falling to the ground. Grosskreutz testified that he feared for his own life, given the presence of Rittenhouse’s rifle, and was trying to disarm Rittenhouse, not kill him. Asked on the witness stand what was going through his mind during their encounter, Grosskreutz replied, “That I was going to die.”
But prosecutors also made repeated missteps, prompting defense lawyers to request a mistrial on two occasions, the first over Assistant District Attorney Thomas Binger questioning Kyle Rittenhouse right to remain silent and a second over a drone video that prosecutors had inadvertently compressed when sharing it with defense lawyers.
And legal experts said prosecutors relied too heavily on video evidence that was difficult to interpret and witnesses who offered testimony that at times seemed to bolster Rittenhouse’s case.
“The prosecution had a very difficult time establishing its own case through its own witnesses. Each of the witnesses that the prosecution put forward did something to help their case but also did something that was harmful to their case,” said Charles Coleman Jr., a civil rights lawyer and former prosecutor.
In the end, jurors were ultimately persuaded by Kyle Rittenhouse version of events.
“Some might have had concerns about the decisions that brought Mr. Kyle Rittenhouse to Kenosha that evening and how he reacted. However, unless the state proves beyond a reasonable doubt that this wasn’t reasonable and that he didn’t believe that he needed to use deadly force to defend himself, then the only proper verdict is acquittal,” said Chris Zachar, a criminal defense attorney based in La Crosse, Wis., who was not involved in the trial.
Rittenhouse could still be sued for damages in a civil trial, where the burden of proof is lower than in criminal trials.
In a statement, Huber’s parents, Karen Bloom and John Huber, said that their son “would have his day in court” and that they were “heartbroken and angry” over the acquittal.
“Today’s verdict means there is no accountability for the person who murdered our son. It sends the unacceptable message that armed civilians can show up in any town, incite violence, and then use the danger they have created to justify shooting people in the street,” they said.
Kyle Rittenhouse : A lightning rod of a trial, broadcast widely across the U.S.

The case has sparked passionate reactions from the American public since the moment Rittenhouse fired his rifle that night.
Nearly every aspect of the case touches on some of the country’s most contentious fault lines: Second Amendment rights, self-defense, violence at racial justice protests, vigilantism and perceptions of how police and the justice system treat white people and people of color differently.
Supporters saw Kyle Rittenhouse as a champion of gun rights who bravely stepped in to protect a community from what they considered lawless riots. Opponents instead saw an irresponsible vigilante who came to Kenosha to play dress-up as a police officer — a “chaos tourist,” as Binger put it.
“What comes to mind is the comment that, ‘How do you stop an active shooter? Good guys with guns.’ In this case, you’ve got Mr. Kyle Rittenhouse, who fires off four shots at somebody who’s unarmed and then continues to shoot people,” said Zachar. “Who’s the good guy? Who’s the bad guy in that scenario? No, nobody really knows.”
For the past two weeks, the trial has commanded the nation’s attention — thanks in no small part to the fact that nearly every minute of the proceedings was broadcast widely on TV and livestream video. Everyone from basketball star LeBron James to Rep. Marjorie Taylor Greene, R-Ga., weighed in before the verdict.
The White House released a statement from President Biden on Friday saying that the verdict “will leave many Americans feeling angry and concerned, myself included,” but that “we must acknowledge that the jury has spoken.” Biden urged people to refrain from violence and property destruction and said he had contacted Wisconsin’s governor to offer “support and any assistance needed to ensure public safety.”
Home Improvement
What Phoenix housing market buyers and sellers can expect in 2023

The Metro Phoenix housing market has heavily favored home sellers since the start of the decade as more people moved to Arizona and the pandemic scrambled the status quo. The S&P CoreLogic Case-Shiller Index’s most recent release shows that home prices in Phoenix rose 17.1% from August 2021 to August 2022. In the past few months, however, the dynamic has started to shift. Andrea Crouch, president of Phoenix REALTORS, notes that prices are beginning to go down.
“It’s simply a supply and demand issue,” she says. “The iBuyers have evacuated here, so that’s left actual owner-occupants to be the buyers, but the increase in interest rates has made it more challenging for them. That’s the reason we’ve seen that dip, but the bottom is not going to fall out by any stretch. It’s just turning into a more normal market, which is just fine.”
One of the indicators that tracks whether the Metro Phoenix housing market is tilted towards buyers or sellers is the Cromford Market Index. Rich La Rue, a broker with HomeSmart, explains that the index takes into consideration business done through the day prior.
“Today’s [Sept. 6] chart is at 105.3,” he says. “100 is considered a perfectly balanced market, but really anything from 90 to 110 Cromford considers balanced.”
In the two months since speaking with La Rue, the Cromford Market Index has slid to approximately 92, approaching buyer’s market territory. As the Greater Phoenix housing market returns to a semblance of normalcy, what does that mean for buyers and sellers as 2023 approaches?
The Phoenix housing market advantage
Stories abound of how in the last two years, would-be buyers faced fierce competition as homes would receive dozens of offers above asking price within days — sometimes hours — of being listed for sale. Trevor Halpern, CEO of Halpern Residential at North&Co., says that intense demand and low supply of homes dramatically increased the power of sellers, but the negotiating disparity is now waning.
“Both buyers and sellers have a reasonable shot at succeeding in this marketplace,” Halpern says. “When [the market is] balanced like this, everyone knows that they’re going to need to come to the table. We see some winners and some losers on both sides, as opposed to six months ago when the buyers were always on the short end of the stick.”
For sellers, Crouch advises that homeowners adjust their expectations to match the change in bargaining position. The days of every home getting multiple offers just because it’s for sale have passed, but she says that people can still fetch a solid price if the right steps are taken, such as having high quality photos of the entire property.
“We’re back to having one opportunity to make a good first impression, so you should make sure your home is show ready and have a trusted real estate professional who will guide you through preparing your home for sale,” Crouch notes.
Since people are no longer willing to pay tens of thousands of dollars over asking price like they were a year ago, La Rue suggests that sellers consider offering concessions such as a home warranty or allowing for an appraisal contingency.
One of the best things buyers can do, according to La Rue, is to get preapproved for a loan because sellers are always looking to reduce uncertainty when putting their house under contract. In a rising interest rate environment, he adds that buyers can flex their bargaining power by asking for an interest rate buydown.
“A simple 2-1 buydown costs the seller 3%,” he says. “I’m using rough numbers here, but what that means for the buyer is instead of having a 5.5% [mortgage] loan the first year, they’re paying 3.5%, then 4.5% the second year and 5.5% for the remainder of the loan.”
Inventory growth
One of the reasons that buyers and sellers are on more equal footing comes down to the supply of homes. According to Realtor.com’s residential listings database, the Phoenix Metro area has risen from 4,688 active for-sale listings in January 2022 to 16,778 as of September 2022 — a 257% increase. The boom in homes available has occurred because of the rising cost of borrowing money, Halpern notes.
“When interest rates rose dramatically over a compressed period of time — from about 3% into the 5% range within 60 days — it was like buyers went on strike. Sellers panicked because they were thinking, ‘We’ve hit the [market] peak so we need to sell right now,’” he says.
Simultaneously, demand sputtered since buyers who were qualified at lower interest rates could no longer afford the monthly payments for the homes they were considering purchasing, so they backed out. This pushed the average time on market up to 40 days, which Crouch says is still relatively low compared to years past.
“When sellers had to sift through 30 offers trying to figure out which one was going to get all the way to closing, buyers had to act so quickly they didn’t have a chance to think,” she says. “The chaos wasn’t good for anyone.”
Tucker Blalock, managing broker & co-founder of The Brokery, has noticed that buyers don’t feel the need to make an offer immediately anymore. “People will come into one of our houses and love it, then say, ‘I’ll get back to you next week.’ There’s no sense of urgency,” he says. “Buyers are exhausting all their options before making a decision now, rather than jumping on the first one they see.”
Even though more homes are being listed, Halpern says that the pace has settled into a more normal range, and that concerns about institutional investors flooding the market with “shadow inventory” are largely unfounded.
“We’ve seen blips of movement, with units changing hands between hedge funds and big companies such as Zillow, but we haven’t experienced a massive dump of properties within our marketplace that would affect pricing on any large scale. They would have to list thousands of homes suddenly to do that,” Halpern says. “Unless [these entities] can figure out an exit that makes sense for their investors, they’re not going to do that because Wall Street will beat them up for it.”
Looking to 2023, no one can foresee whether home prices will rise or fall, but Blalock advises that people should focus on the primary function of their home and try to ignore the instinct to time the market — something he and Halpern both agree is unwise if not impossible.
“Don’t think that the real estate market moves like the stock market. While it has been more volatile over the past couple of years, things will normalize as they have over the past few months,” Blalock concludes. “Keep a longer-term approach, especially when it comes to your primary residence, which should be looked at as somewhere to live and enjoy, not strictly as an investment. If you’re comfortable with the payments you’re making on a monthly basis, there’s no reason to fret.”
Home Improvement
Portfolio Points: Essential Tips To Invest In Real Estate Profitably

Real estate investing is a great way to make money for your portfolio. The best part is that you don’t have to have a lot of capital to start investing. Using the tips below, you can start investing in real estate and become a successful real estate investor.
Learn The Basics of Real Estate Investing
The first step in becoming a successful real estate investor is to learn the basics of real estate investing. Investing in real estate requires knowledge of the market, how to find deals, how to negotiate with sellers, and how to buy property. Here are some tips on how to get started:
- Look for a mentor. A mentor can help you learn the basics of real estate investing. Ask friends, family members, or even your bank manager to recommend a mentor in your area.
- Build your real estate portfolio. Before you invest in real estate, you should have a portfolio of properties that you are comfortable with. Once you have a portfolio, you can begin investing in real estate.
- Attend real estate seminars. Real estate seminars are a great way to learn the latest trends in real estate investing. As an investor, you should always be on top of the latest trends.
- Read books on real estate investing. There are many books on real estate investing that can help you to learn more about investing in real estate.
- Use online resources. Online resources are a great way to learn about investing in real estate. You can learn about the latest trends, find out about new tools that are available, and even find out about local deals in your area.
Invest in Funds and Trusts With A Low DSCR
When you invest in funds and trusts, you need to make sure that the DSCR is low. The DSCR is what determines how much money is needed for debt servicing costs. If the DSCR is too high, it can limit your ability to make money as an investor. You need to make sure that the DSCR is under 2% so that you can continue to make money as an investor.
Determine Your Risk Tolerance
Before you invest in real estate, you need to determine your risk tolerance. This means that you need to determine if you are willing to take on risks as an investor. For example, if you want to invest in properties in a high-risk area, then you may not want to invest in real estate. However, if you want to invest in a lower-risk area, then investing in real estate may be right for you.
Understand Your Return Expectancy
To determine your return expectancy, you need to consider several factors. First, you need to consider the return on investment (ROI). You need to determine if the ROI will be enough to cover your costs and make a profit for yourself. Second, you need to consider the time frame that you will be investing in real estate. The longer the time frame, the higher the return expectancy will be. And lastly, you need to consider your investment strategy. The more diversified your investments, the higher the return expectancy will be.
Get the Tools You Need to Make Money
As an investor, you need to get the proper tools that you need to make money as an investor. This will include access to a computer and internet connection so that you can find deals online and access other online tools for finding deals in your area. You should also have access to software that will help you decide what properties are good investments and what properties are not good investments. Finally, you should have access to training so that you can learn more about investing in real estate and how to find deals online and offline.
Lower Your Investment Expenses
One of the biggest mistakes that people make when they start investing in real estate is that they pay too much for their properties. When you buy properties, you should take into consideration how much it costs you per month just to own that property. You need to compare this cost with how much money your property will bring in each month and determine if it is worth it for that property or not. If it isn’t worth it, then sell it and move on to another property. If it is worth it, then keep it and continue making money as an investor.
Make Time for Home Improvement Projects
The best way for you to make money as an investor is by making time for home improvement projects. When you do home improvement projects, you can make money for yourself by selling the materials that you used for these projects after the project is completed and by selling the finished product once it’s done. You can also use these materials to sell at a later date if needed. There are also tax benefits when selling these materials as well as cash flow benefits from using these materials for home improvement projects. It’s important to understand these benefits before beginning any project so that you can maximize your profits as an investor.
Learning about real estate investing can be difficult at first because there is so much information out there about how it works. However, once you understand how the system works and how investors make money, then it becomes easier for you too. All it takes is some determination and hard work on your part and everything will fall into place for you as an investor.
-

 News2 years ago
News2 years agoDr. Naval Parikh: Hypertension Types, Causes, Symptoms & Treatment
-

 News2 years ago
News2 years agoDr. Naval Parikh: Allergy Types, Causes, Symptoms & Treatment
-

 News2 years ago
News2 years agoDr. Naval Parikh: Asthma Causes, Symptoms and Treatment Options
-

 Celebrity4 years ago
Celebrity4 years agoYoung Irish Star Signs to DJ John Gibbons’ label
-

 News3 years ago
News3 years ago8 Best Sites Like VIPLeague to Watch Free Sports in 2021
-

 News1 year ago
News1 year agoTinyZone: WatchFree Movies Online
-

 News2 years ago
News2 years agoTampa Van Hire
-

 Uncategorized2 years ago
Uncategorized2 years agoInstagram’s Most Popular Product Niche
